Factory Supply Disposable Arterial Venous Fistula Needle Set - Plasmapheresis Centrifuge Apparatus For Single Use – Zhongbaokang Medical
Short Description:
Product Detail
Product Tags
Related Video
Feedback (2)
Factory Supply Disposable Arterial Venous Fistula Needle Set - Plasmapheresis Centrifuge Apparatus For Single Use – Zhongbaokang Medical Detail:
Device description
1、 Device Name:Plasmapheresis Centrifuge Apparatus for Single Use
2、Model: ZXLQ-350 type
Device main structure and application scope
(1) Device description
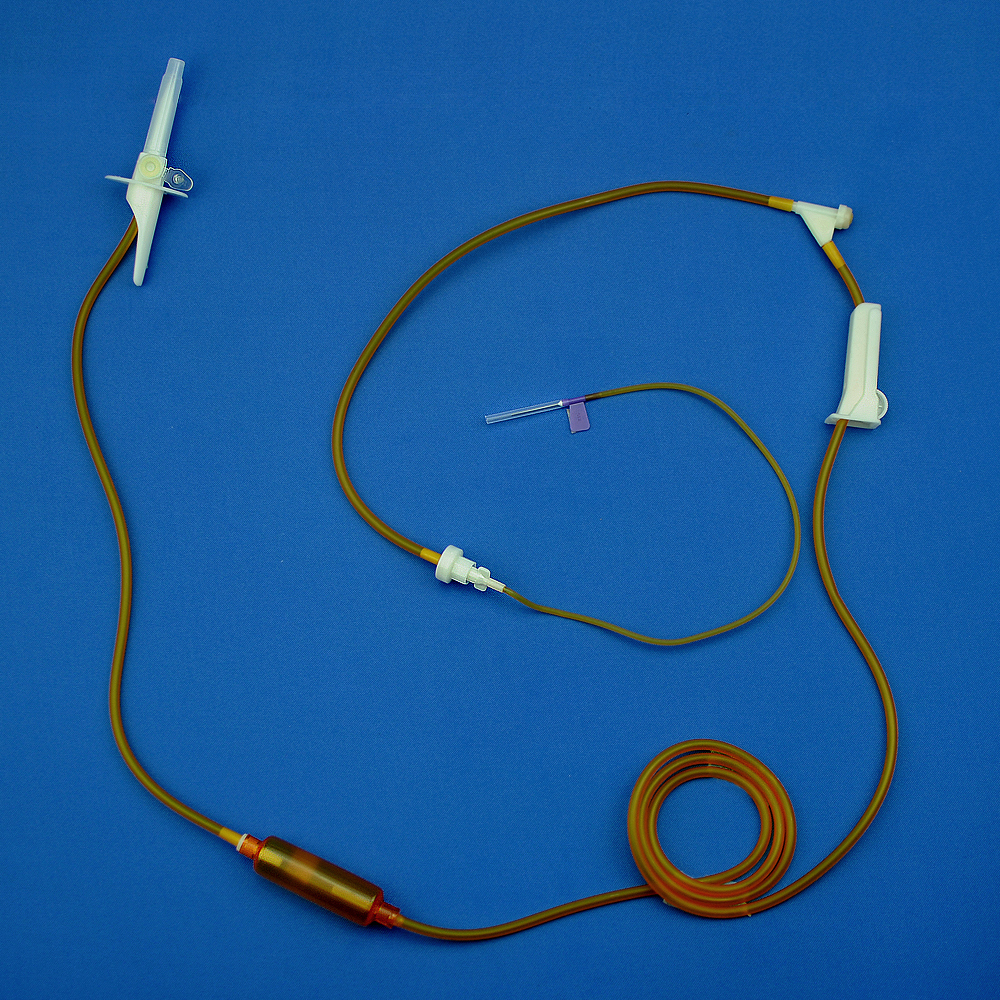
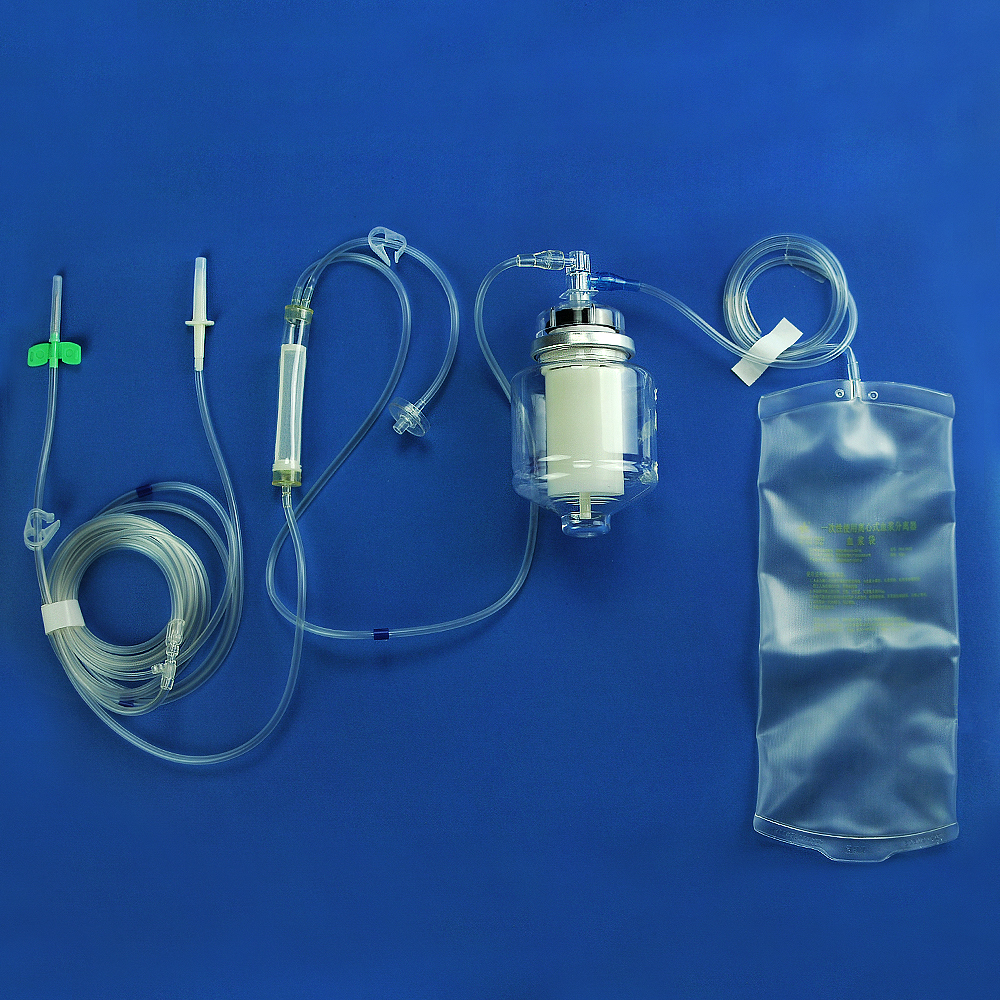
The plasmapheresis centrifuge apparatus for single use consists of a plasmapheresis cup, tubing, A. V. fistula needle sets and plasma bag.(This product does not contain anticoagulant)
Device description:
1. Protective Cap for Closure-piercing Device (Plastic Needle)
2. Closure-piercing device 3. Anti-freezing Liquid Tubing
4. Block 5. Three-Way Cock
6. Blood Collection & Transfusion Tube 7. Filter for Blood & Blood Component
8. Fixture 9. Pressure Monitor Joint
10. Plasmapheresis Cup Joint 11. Plasmapheresis Cup
12. Plasma Bag 13. A. V. fistula needle sets
Figure 1 Schematic of Plasmapheresis Centrifuge Apparatus for Single Use
(2) The plasmapheresis centrifuge apparatus for single use is used in conjunction with Haemonetics PCS2 automated plasma collection system for the collection of human blood plasma and transfusing the red blood cell back to the blood donor.
Classification:Class IIb
The classification standard conforms to the Section III, Appendix IX of the MDD of 93/42/EEC as amended by 2007/47/EC.
Contraindications
(1) Hemodynamic instability, sepsis, or other conditions causing inability to tolerate fluid shifts.
(2) Severe hypocalcemia (citrate anticoagulant will precipitate calcium and worsen hypocalcemia)
Precautions
(1) Do not use the product if its protective cap of A. V. fistula needle sets falls off.
(2) In the operation, operators must wear gloves and not contact the blood directly. If the blood bag is broken, disinfectant is required for sterilization to prevent the operator from being polluted.
Warnings
(1) Donor eligibility MUST be evaluated strictly in accordance with accepted medical practice and applicable local and national requirements.
(2) Read these instructions completely prior to use.
(3) Contents are sterile unless package is opened or damaged. For single use only. Discard any open, unused product Do not use after the expiration date.
(4) The product contains DEHP (Bis(2-ethylhexyl)phthalate). Based on animal studies, significant exposure to DEHP may interfere with the normal development of the male reproductive tract. For children, pregnant and nursing women or any other patient group considered particularly vulnerable or exposed to high levels of phthalates, alternative devices shall be selected for higher priority.
(5) Do not resterilize the device.
(6) Do not reuse the product. Reuse of the device may potentially result in serious patient harm. Examples of hazards related to the reuse of the devices include, but are not limited to: significant deterioration in device performance, cross-infection and contamination.Ÿ
Direction for Use
(1) Open the packing bag on a clean bench along the line where the word “incision” is printed.
(2) Install the device with Haemonetics PCS2 and select appropriate procedure setting at the PCS2 system. Refer to the Operating Manual of the Haemonetics PCS2 for detailed instruction.
(3) Optional: When required, connect the Pressure Monitor Joint with pressure monitoring device.
(4) Prior to initiating a procedure, perform a single venous puncture.
(5) Initiate the selected procedure at Haemonetics PCS2 machine. Plasma collection will proceed automatically until the end-collection target has been reached.
(6) Disconnect the plasma bag from the tubing set, and send it for refrigeration as requested for follow-up application.
(7) After use, this device may be a potential biohazard and should be handled in accordance with accepted medical practice and applicable local and national requirements.
Storage
The product shall be stored in a dry and ventilated place, away from corrosive gases. And the packing box shall be identified with words, like fragile, keeping away from wet and heat, and limits of stacking, etc. The storage temperature is recommended to be within 5~24℃.
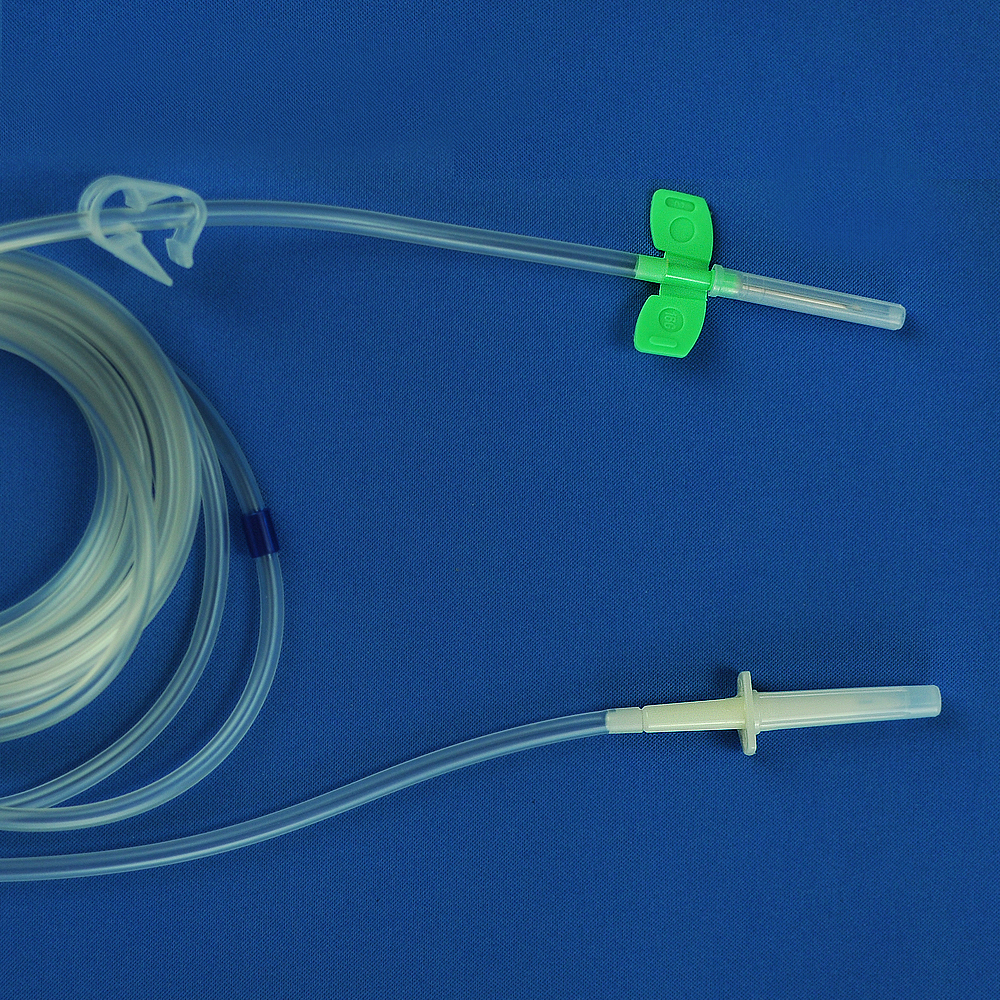
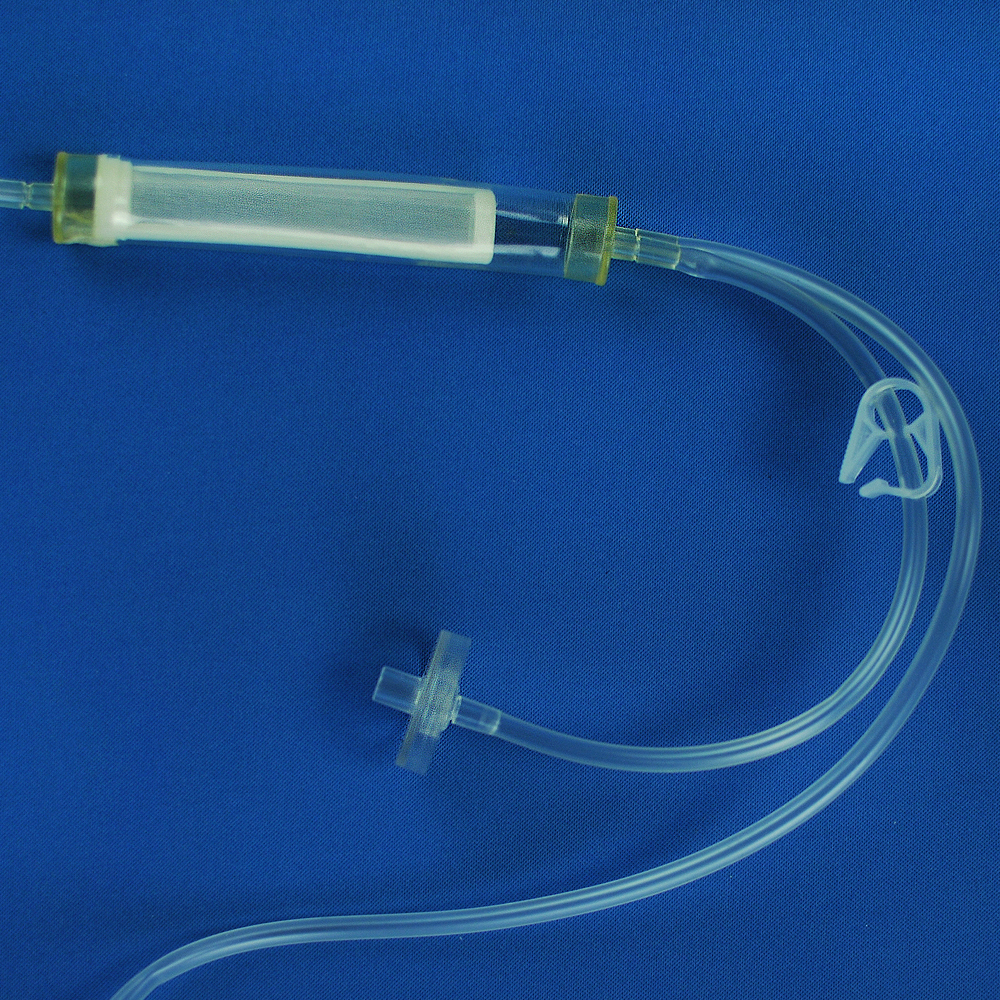
Product detail pictures:

Related Product Guide:
Inhaled version of blood pressure drug shows promise in treating anxiety, pain | White Cells Filter
NIRI wins award for universal plasma project | White Cells Filter
Factory Supply Disposable Arterial Venous Fistula Needle Set - Plasmapheresis Centrifuge Apparatus For Single Use – Zhongbaokang Medical, The product will supply to all over the world, such as: , , ,