“I’m sorry to hear that. Very sorry. Nothing like that happened to me. The only thing remotely connected to something like that is that the windshield of my car was shamed with a hammer. But that’s just a city thing. I park outside.”
At the end, this Automotive Diesel Filters market report provides a comprehensive study that takes account of the historical data, presents the current state, and anticipates the future. Also, this report includes extremely useful information for the new and growing company to mark themselves over the market. Automotive Diesel Filters market report also contains important details such as End Users/Application, Trends in Future, Status, and Outlook, production capacity, revenue, and Scope.
If your main concern is softening water to make it gentler on your hair and skin, George says this filter “does the best job at purifying shower water. It has two powerful stages of filtration [and] enhances the water pH balance.” Redox and activated carbon remove chlorine and heavy metals, and neutralize potential contaminants.
The answer, at one level, is simple. India’s family laws allow for divorce but they also allow husbands to walk out of a marriage without completing the formalities of divorce. And that’s not right, surely.
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties relating to future events and the future performance of Alexion, including statements related to: ULTOMIRIS has the potential to be the new standard of care for patients with paroxysmal nocturnal hemoglobinuria (PNH); ULTOMIRIS can provide meaningful benefits for patients with PNH and their families; Alexion will work with regulatory authorities in certain jurisdictions to enable timely review of applications for approval of ULTOMIRIS as a treatment for adults with PNH; Alexion plans to initiate a Phase 3 clinical study of ULTOMIRIS delivered subcutaneously once per week as a potential treatment for patients with PNH and atypical hemolytic uremic syndrome (aHUS); Alexion is planning to initiate the development of ULTOMIRIS, intravenously administered every eight weeks, as a potential treatment for patients with generalized myasthenia gravis (gMG); planned future studies of ULTOMIRIS for other indications; and the potential medical benefits of ULTOMIRIS for the treatment of PNH and other diseases. Forward-looking statements are subject to factors that may cause Alexion’s results and plans to differ materially from those expected by these forward looking statements, including for example: ULTOMIRIS does not gain market acceptance and/or is not recognized by patients and physicians as the standard of care for patients with PNH; the benefits (including safety and efficacy) of ULTOMIRIS evidenced in clinical trials are not witnessed in a broader patient population; any potential post-approval restrictions that the FDA may impose on ULTOMIRIS; our dependence on sales from our principal product (SOLIRIS); future competition from biosimilars and other products; decisions of regulatory authorities regarding the adequacy of our research, marketing approval or material limitations on the marketing of our products; delays or failure of product candidates to obtain regulatory approval; delays or the inability to launch product candidates due to regulatory restrictions, anticipated expense or other matters; interruptions or failures in the manufacture and supply of our products and our product candidates; failure to satisfactorily address matters raised by the FDA and other regulatory agencies; results in early stage clinical trials may not be indicative of full results or results from later stage or larger clinical trials (or broader patient populations) and do not ensure regulatory approval; the possibility that results of clinical trials are not predictive of safety and efficacy and potency of our products (or we fail to adequately operate or manage our clinical trials) which could cause us to halt trials, delay or prevent us from making regulatory approval filings or result in denial of approval of our product candidates; unexpected delays in clinical trials; future product improvements may not be realized due to expense or feasibility; uncertainty of long-term success in developing, licensing or acquiring other product candidates or additional indications for existing products; inability to complete planned acquisitions due to failure of regulatory approval or material changes in the target or otherwise; inability to complete acquisitions and investments due to increased competition for technology; the possibility that current rates of adoption of SOLIRIS in PNH, aHUS, gMG or other diseases are not sustained; the adequacy of our pharmacovigilance and drug safety reporting processes; failure to protect and enforce our data, intellectual property and proprietary rights and the risks and uncertainties relating to intellectual property claims and challenges against us (including intellectual property lawsuits relating to ULTOMIRIS brought by third parties against Alexion); the risk that third party payers (including governmental agencies) will not reimburse or continue to reimburse for the use of our products at acceptable rates or at all; failure to realize the benefits and potential of investments, collaborations, licenses and acquisitions; delay of collection or reduction in reimbursement due to adverse economic conditions or changes in government and private insurer regulations and approaches to reimbursement; uncertainties surrounding legal proceedings (including intellectual property suits initiated against Alexion and our products), company investigations and government investigations, including investigations of Alexion by the U.S. Securities and Exchange Commission (SEC) and U.S. Department of Justice; the risk that estimates regarding the number of patients with PNH, aHUS, gMG, HPP and LAL-D and other future indications we are pursuing are inaccurate; the risks of changing foreign exchange rates; risks relating to the potential effects of the Company’s restructuring; risks related to the acquisition of Syntimmune and other companies and co-development efforts; and a variety of other risks set forth from time to time in Alexion’s filings with the SEC, including but not limited to the risks discussed in Alexion’s Quarterly Report on Form 10-Q for the period ended September 30, 2018 and in our other filings with the SEC. Alexion disclaims any obligation to update any of these forward-looking statements to reflect events or circumstances after the date hereof, except when a duty arises under law.
"That means that people who were involved with the burial rituals had to help the soul move on,” he says.
environmental engineer A person who uses science to study and solve problems in ecosystems — from forests to the human body.
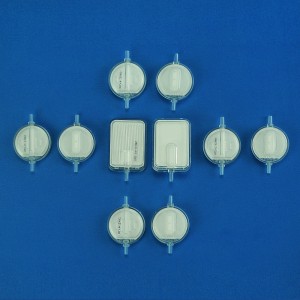
Globally the plasma separation tube market is segmented on the basis of applications and end-use industry which are further segmented as –

Eliminating the need to burn stuff in a house – gas or otherwise – does eliminate the possibility of CO poisoning in that house. It would be pretty difficult to get CO in there otherwise.
Enter your email address to subscribe to The Kashmir Monitor and receive notifications of new stories by email.
That hotels globally do not have mandates to have these sensors deployed is insane. You’d think from an insurance standpoint they would have had them years ago.
In other words, once the law is passed, a rotten Muslim husband who wants to abandon his wife will simply do what his rotten Hindu, Christian, Jain, Buddhist and Sikh counterparts do – kick her out of the marital home.
Regulating IV infusion with innovative blind cave fish-inspired sensor | Wholesale Leukocyte Filter Price Related Video:
, , ,
